Matter is the substance that makes up everything in the universe, from the air we breathe to the ground beneath our feet. At its core, matter is composed of atoms and molecules, and these particles organize themselves in various ways to create the different states of matter. Traditionally, there are four fundamental states of matter: solid, liquid, gas, and plasma. However, modern science has revealed additional states, such as Bose-Einstein condensates and fermionic condensates, which occur under extreme conditions.
This guide explores the states of matter, their properties, transitions, and real-world applications. Understanding the states of matter is essential for grasping the principles of chemistry, physics, and material science, as well as appreciating the natural phenomena around us.
What Are the States of Matter?
States of matter refer to the distinct forms that matter can take, depending on the arrangement and energy of its particles. The most familiar states—solid, liquid, and gas—are encountered daily, while plasma and other exotic states are typically observed under specialized conditions.
- Solid
- Properties: Solids have a definite shape and volume. Their particles are closely packed in an orderly arrangement, allowing only vibrations in place.
- Examples: Ice, metals, and rocks.
- Key Feature: Strong intermolecular forces keep particles fixed in position.
- Liquid
- Properties: Liquids have a definite volume but take the shape of their container. Their particles are less tightly packed than in solids and can slide past one another.
- Examples: Water, oil, and alcohol.
- Key Feature: Intermolecular forces are weaker than in solids, allowing fluidity.
- Gas
- Properties: Gases have neither a definite shape nor volume. They expand to fill their container and can be compressed.
- Examples: Oxygen, nitrogen, and carbon dioxide.
- Key Feature: Particles move independently with negligible intermolecular forces.
- Plasma
- Properties: Plasma consists of ionized gas, where electrons are separated from their nuclei, creating a soup of charged particles.
- Examples: Lightning, stars, and neon signs.
- Key Feature: Highly energetic particles make plasma conductive and reactive.
- Exotic States:
- Bose-Einstein Condensates: Formed at temperatures close to absolute zero, where particles coalesce into a single quantum state.
- Fermionic Condensates: Similar to Bose-Einstein condensates but occur with fermions under specific conditions.
Particle Behavior in Different States
The behavior of particles is the defining feature of each state of matter. The arrangement, motion, and interaction of particles vary significantly across states:
- In solids, particles are tightly packed in a fixed arrangement, vibrating in place but not moving freely.
- In liquids, particles have more energy, allowing them to slide past one another while remaining close.
- In gases, particles have enough energy to move independently, filling the available space.
- In plasma, the high energy levels cause particles to ionize, resulting in free electrons and ions.
These differences explain why a block of ice maintains its shape, water flows, and steam disperses rapidly.
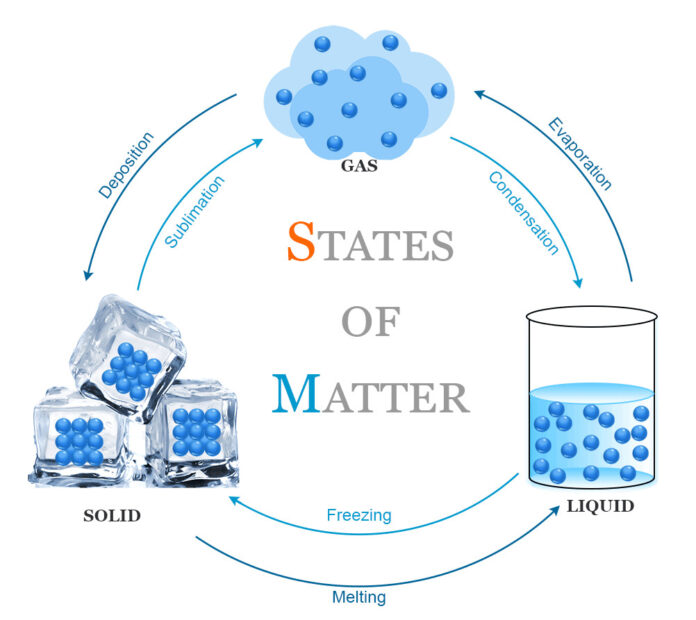
Changes Between States: Phase Transitions
Matter can transition between states through phase changes, driven by changes in temperature or pressure. These transitions include:
- Melting: The process of a solid becoming a liquid as it absorbs heat. For example, ice melts into water at 0°C.
Freezing: The reverse of melting, where a liquid becomes a solid as it loses heat. Water freezes into ice below 0°C.
\(H_2O_{\text{(l)}} \rightarrow H_2O_{\text{(s)}}\)Vaporization: A liquid becomes a gas, either through boiling or evaporation. Water boils at 100°C at standard atmospheric pressure.
\(H_2O_{\text{(l)}} \rightarrow H_2O_{\text{(g)}}\)Condensation: The reverse of vaporization, where a gas turns into a liquid as it cools. Water vapor condenses on a cold surface.
\(H_2O_{\text{(g)}} \rightarrow H_2O_{\text{(l)}}\)Sublimation: A solid transitions directly to a gas without passing through the liquid phase, as seen in dry ice.
\(CO_2\_{\text{(s)}} \rightarrow CO_2\_{\text{(g)}}\)Deposition: The reverse of sublimation, where a gas turns directly into a solid. Frost forming on a surface is an example.
\(H_2O_{\text{(g)}} \rightarrow H_2O_{\text{(s)}}\)Ionization and Recombination: In plasma, ionization separates electrons from atoms, while recombination restores them.
Energy and States of Matter
The state of matter depends on the energy of its particles:
- Low Energy: Particles are tightly bound, forming solids.
- Moderate Energy: Particles gain enough energy to move, forming liquids.
- High Energy: Particles move freely, forming gases.
- Very High Energy: Particles ionize, forming plasma.
Energy changes during phase transitions are represented by latent heat:
- Latent Heat of Fusion: Energy required to melt a solid.
- Latent Heat of Vaporization: Energy required to vaporize a liquid.
For example, the energy required to vaporize water can be calculated using: \(Q = m \times L_{\text{v}}\)
Where:
- \(Q\) is the heat energy,
- \(m\) is the mass,
- \(L_{\text{v}}\) is the latent heat of vaporization.
Real-World Applications of States of Matter
Understanding states of matter and their transitions is crucial in numerous fields:
- Industrial Applications
- Refrigeration: Phase transitions between liquid and gas in refrigerants cool homes and preserve food.
- Steel Production: Transition between solid and liquid states is essential for molding and shaping metals.
- Medical Applications
- Cryogenics: Liquid nitrogen is used to preserve biological samples and perform surgeries.
- Plasma Therapy: Plasma is used to sterilize surgical tools and treat wounds.
- Astronomy
- Plasma in Stars: The sun and other stars are composed primarily of plasma.
- Interstellar Ice: Solid-state ice exists in space under extreme conditions.
- Everyday Life
- Water boiling for cooking involves vaporization.
- Dew forming on grass involves condensation.
Exotic States of Matter
Recent advancements in physics have revealed states of matter beyond the traditional four:
- Bose-Einstein Condensates (BECs)
- Formed at temperatures close to absolute zero.
- Particles lose individuality, behaving as a single quantum entity.
- Fermionic Condensates
- Similar to BECs but involve fermions instead of bosons.
- Superfluidity
- Occurs in helium at extremely low temperatures, allowing it to flow without viscosity.
- Quark-Gluon Plasma
- High-energy state where quarks and gluons, the building blocks of protons and neutrons, exist freely.
The states of matter are a fascinating aspect of the natural world, governing how substances interact, transform, and exist. From the rigidity of solids to the fluidity of liquids, the freedom of gases, and the high-energy dynamics of plasma, each state reveals a unique facet of matter’s behavior. Modern research into exotic states continues to push the boundaries of what we know, offering deeper insights into the universe’s workings. Understanding states of matter is not only a cornerstone of science but also a gateway to technological innovation and the exploration of nature’s mysteries.