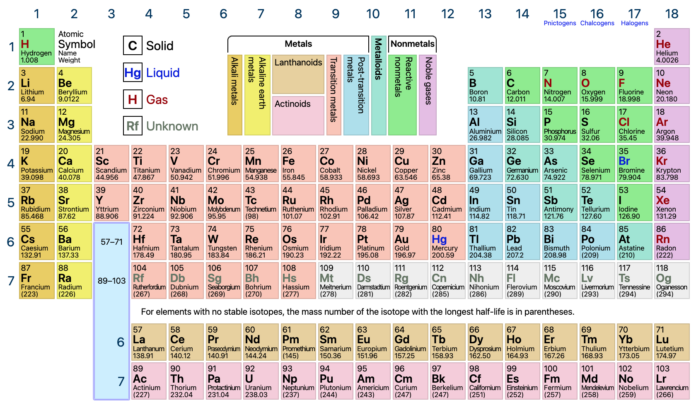
The periodic table is one of the most significant achievements in science, serving as a roadmap for understanding the elements that make up our universe. It organizes all known chemical elements in a logical and systematic way, revealing patterns in their properties and behaviors. From its origins in the 19th century to its modern form, the periodic table has become an indispensable tool in chemistry, physics, biology, and engineering. This article explores the history, structure, and significance of the periodic table, as well as its role in scientific discovery and education.
Origins of the Periodic Table
The concept of organizing elements dates back to the early 19th century when scientists began discovering and classifying elements based on their properties. By the mid-1800s, the number of known elements had grown significantly, prompting the need for a systematic arrangement.
In 1869, Russian chemist Dmitri Mendeleev published the first version of the periodic table. Mendeleev arranged elements by increasing atomic weight and grouped them based on similar chemical properties. His groundbreaking work predicted the existence and properties of elements yet to be discovered, such as gallium and germanium. These predictions were later confirmed, solidifying the periodic table as a powerful scientific tool.
Mendeleev’s table was not the only attempt at organizing the elements, but it stood out for its accuracy and predictive power. Over time, the table was refined as new elements were discovered and atomic theory advanced.
Modern Periodic Table
The modern periodic table, refined by scientists like Henry Moseley, organizes elements by increasing atomic number rather than atomic weight. The atomic number represents the number of protons in an atom’s nucleus, which uniquely identifies each element. This arrangement resolved inconsistencies in Mendeleev’s table and provided a more accurate representation of periodic trends.
Today, the periodic table consists of 118 confirmed elements, arranged in rows (periods) and columns (groups or families). Each element is represented by a unique chemical symbol, such as H for hydrogen or O for oxygen, and includes information about its atomic number, atomic mass, and electron configuration.
Structure of the Periodic Table
The periodic table’s structure is both logical and elegant, designed to highlight periodic trends and relationships among elements.
- Periods
The rows of the periodic table are called periods. Elements within the same period have the same number of electron shells but differ in the number of electrons and protons. As you move from left to right across a period, properties such as electronegativity and ionization energy generally increase. - Groups
The columns of the periodic table are called groups or families. Elements within the same group share similar chemical properties due to having the same number of valence electrons. For example:- Group 1: Alkali metals, which are highly reactive and include elements like sodium (Na) and potassium (K).
- Group 2: Alkaline earth metals, such as calcium (Ca) and magnesium (Mg).
- Group 17: Halogens, highly reactive nonmetals like chlorine (Cl) and fluorine (F).
- Group 18: Noble gases, known for their stability and lack of reactivity, including helium (He) and neon (Ne).
- Transition Metals
Found in the middle of the table, transition metals such as iron (Fe), copper (Cu), and gold (Au) are known for their versatility and ability to form complex compounds. - Lanthanides and Actinides
These two rows at the bottom of the periodic table include elements with unique properties. Lanthanides are often used in technology, such as rare-earth magnets, while actinides include radioactive elements like uranium (U) and plutonium (Pu).
Periodic Trends
The periodic table reveals recurring patterns, or trends, in the properties of elements. Understanding these trends helps scientists predict how elements will behave in chemical reactions.
- Atomic Radius
Atomic radius decreases across a period due to increasing nuclear charge, which pulls electrons closer to the nucleus. It increases down a group as additional electron shells are added. - Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, increases across a period and decreases down a group. Elements with high ionization energy, like noble gases, are less likely to lose electrons. - Electronegativity
Electronegativity, the tendency of an atom to attract electrons, increases across a period and decreases down a group. Fluorine is the most electronegative element. - Reactivity
Reactivity varies across the table. Alkali metals are highly reactive due to their single valence electron, while noble gases are inert. Halogens are reactive nonmetals, often forming compounds with alkali metals.
Applications of the Periodic Table
The periodic table is a cornerstone of scientific research and education. It provides a framework for understanding chemical reactions, material properties, and biological processes.
- Chemistry and Material Science
The periodic table guides chemists in predicting reactions, designing new compounds, and studying the properties of materials. For example, understanding the properties of transition metals has enabled advancements in catalysis and metallurgy. - Biology and Medicine
Elements like carbon, hydrogen, nitrogen, and oxygen are essential to life, forming the basis of organic molecules. Trace elements such as iron and iodine play critical roles in biological processes, such as oxygen transport and thyroid function. - Technology and Industry
The periodic table has driven innovations in technology, from semiconductors to renewable energy. Elements like silicon (Si) are essential for electronics, while rare-earth elements are critical for batteries and magnets. - Environmental Science
Understanding elements and their compounds is key to addressing environmental challenges. For instance, studying carbon dioxide (CO₂) and methane (CH₄) is vital for combating climate change.
The Future of the Periodic Table
As scientific research advances, the periodic table continues to evolve. The discovery of new elements, particularly superheavy elements beyond atomic number 118, challenges our understanding of chemistry and nuclear physics. Efforts to synthesize and study these elements provide insights into the limits of the periodic table and the forces that govern atomic structure.
In addition, the periodic table remains a powerful educational tool, inspiring students and researchers to explore the fundamental building blocks of matter.
The periodic table is more than just a chart of elements; it is a testament to human ingenuity and curiosity. By organizing the building blocks of the universe into a coherent system, the periodic table has revolutionized our understanding of matter and its interactions. From its origins in the 19th century to its role in modern science, the periodic table continues to be an essential tool for discovery and innovation. As we uncover new elements and deepen our understanding of atomic behavior, the periodic table will remain a cornerstone of scientific progress for generations to come.